|
|
|
|||||||||||||||
Phase Diagrams |
|||||||||||||||||
 |
|||||||||||||||||
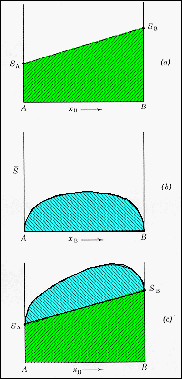
· The Gibbs function curves are composed from entropy and enthalpy contributions. |
|||||||||||||||||
From: Brophy, Rose, & Wulff, "Thermodynamics of Structure," Wiley (1964) |
|||||||||||||||||
|
|
|
|||||||||||||||
Phase Diagrams |
|||||||||||||||||
 |
|||||||||||||||||
· The Gibbs function curves are composed from entropy and enthalpy contributions. |
|||||||||||||||||
From: Brophy, Rose, & Wulff, "Thermodynamics of Structure," Wiley (1964) |
|||||||||||||||||