|
|
|
||||||||||||
Kinetics and Microstructural Control |
||||||||||||||
· The diagram illustrates a spherical
β-nucleus in a homo-geneous α-liquid. |
||||||||||||||
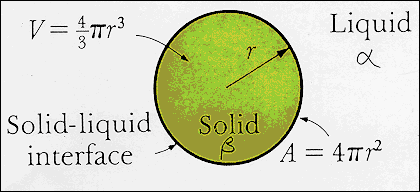 |
||||||||||||||
· The surface
energy associated
with the formation of the beta-phase is given by: ΔGS = 4πr2 γαβ
,
where γαβ = Interfacial Surface
Energy/Unit Area. |
||||||||||||||