|
|
|
|||||||||||||||
| Kinetics and Microstructural Control | |||||||||||||||||
| ·
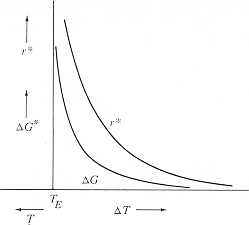
The
temperature dependence of r* and ΔG*
comes from the temperature dependence of ΔGV:
ΔGV
= (Gβ
- Gα)
= ΔHV
- T ΔSV.
· Both the enthalpy and entropy terms are slow functions of temperature and may be evaluated at the equilibrium temperature, TE. · At TE , ΔGV = 0 and ΔHV = TE ΔSV , so that: ΔSV
= ( ΔHV
/ TE ).
|
|||||||||||||||||
 |
|||||||||||||||||
| From:
Barrrett, Nix & Tetelman,
"The Principles of Engineering Materials," Prentice Hall (1973) |
|||||||||||||||||
| ·
Using
this value at all temperatures gives:
ΔG* = {16πγαβ3 / 3ΔHV2 }[ TE/ (TE - T)]2, r* = -{2γαβ / ΔHV}[ TE / (TE - T )] |
|||||||||||||||||