| Corrosion & Environmental Degradation |
|
||||||||||||||||
|
|
|
|||||||||||||||
| ·
The
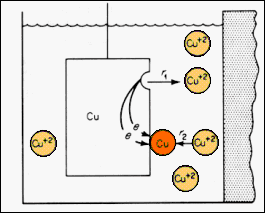
diagram illustrates the steady state behavior of a "reversible electrode"
in contact with a molar concentration of an electrolyte of its ions. Both
the forward and reverse reactions are taking place at the same rate and:
Cu <-> Cu2+ + 2e
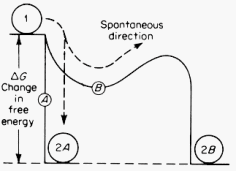
·The configuration represents a "standard half cell" for measuring the electrode potential of this material. A salt bridge is used to make electrical connection between this electrolyte and the electrolyte for the hydrogen reference electrode. ·In establishing this steady state situation, a change in the Gibbs free energy of the system, DG, occurs. If n is the number of electrons involved in establishing the equilibrium condition and the electrode potential is V, then: DG = - nFV where F is the Faraday constant (9.65 x 104 Coulombs per mole of electrons), the process occurring at constant (p, T). DG is path independent as G is a thermodynamic parameter. |
|||||||||||||||||
 |
|||||||||||||||||
| From:
M.G. Fontana,
"Corrosion Engineering," Wiley (1986) |
|||||||||||||||||
 |
|||||||||||||||||